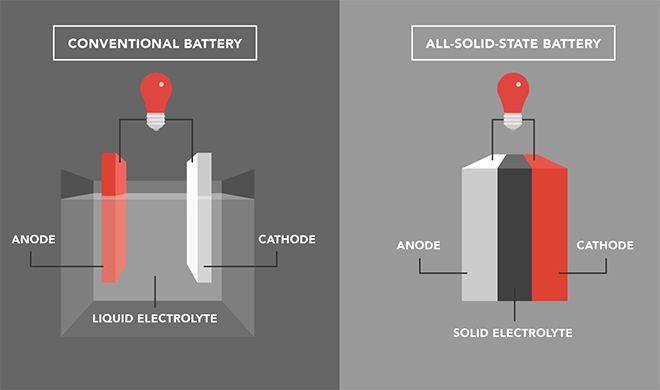
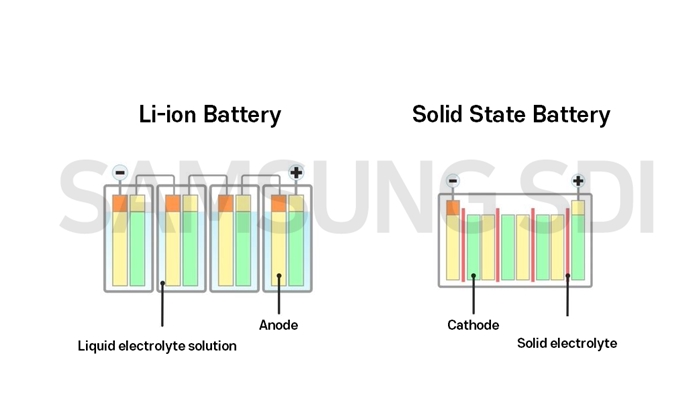
As the influx of electric vehicles is booming, automakers and tech companies have been focusing on optimizing an EV’s most vital and expensive part: the battery. And while lithium-ion batteries have been the mainstream option so far, there’s another promising competitor on the making: solid-state batteries. What do they have in common? Both types use lithium to produce electrical energy and their overall structure is quite similar. Simply put, they have an anode (the battery’s negative side), a cathode (the battery’s positive side), and an electrolyte. How do they differ? Their main difference lies on the state of the electrolyte, which helps to transfer ions from the cathode to the anode when charging, and vice versa when discharging. In other words, the electrolyte regulates the flow of the electric current between the negative and the positive sides of the battery. While lithium-ion batteries use liquid electrolytes, solid-state batteries feature — as their name suggests — thin layers of solid electrolytes. Why does this matter? Solid electrolytes come with a handful of significant advantages.
- Safety Liquid electrolytes are volatile and flammable in high temperatures. In contrast, solid ones are more stable and reduce the risk or fire or explosion.
- Higher energy density and faster charging times Increased stability means that solid-state batteries can hold up to 50% more energy that their lithium-ion counterparts, while they’re expected to reach an 80% charge within 12 minutes.
- Smaller weight and size While the liquid inside lithium-ion batteries makes them heavier, the compact structure of solid-state batteries allows them to increase energy density per unit area, which means that a smaller amount of batteries is needed. Will solid-state batteries replace the lithium-ion ones? In theory, yes, or at least this is where things seem to be going. In fact many automakers are already investing in the tech, including Volkswagen, Toyota, Ford, and BMW. In practice, however, solid-states battery cells have been produced one at a time in labs, and to scale this up to mass production is an expensive and still under-development endeavor. It is difficult to design a solid electrolyte that is at the same time stable, chemically inert, and a good conductor of ions between the electrodes. Plus, the electrolytes are expensive to produce, and prone to cracking because of their fragility when they expand and contract during use. But perhaps, just as lithium-ion batteries became gradually more affordable, the idea is that the solid-state version will be, too. HT – J.D. Power, Samsung SDI, The Harvard Gazette, Reuters Do EVs excite your electrons? Do ebikes get your wheels spinning? Do self-driving cars get you all charged up? Then you need the weekly SHIFT newsletter in your life. Click here to sign up.